Surface Plasmon Resonance: Technology Overview and Practical Applications
by Greg Emmerich, UW Madison, M.S. in Biotechnology Program, Early Drug Development Class. November 16, 2012
Abstract
There is a remarkable moment in science that is best described as the “aha!” moment–the moment when some truth about the universe becomes revealed through precise measurement, observation, and deduction. A seemingly unlikely union between mathematics, physics, and biochemistry has given rise to a technology called Surface Plasmon Resonance (SPR). The “aha!” moment for SPR came some 30 years ago when it was shown that polarized laser light shined upon a nanolayer of gold at a specific frequency can propagate that specific energy along the surface of the gold, and this propagation is extremely sensitive to any changes to the dielectric environment of the gold. That is, lasers were found to be useful as a lens to the world of the very small, able to detect molecular changes on the nanometer scale. The implications of this discovery continue to be felt as companies continue to improve the technology and tailor it to particular needs. Already SPR has proven useful in the drug discovery and molecular diagnostic fields, and the technology shows much promise in the future.
Introduction
Advancement of medicinal science has largely relied upon molecular techniques to understand disease physiology, screen compounds against a disease target, and then optimize any leads before proceeding to preclinical in vivo testing. This process is known as drug discovery, and it is a very long and expensive process. Pharmaceutical companies are always on the lookout for any new technologies that can increase efficiency and reduce cost when developing new drugs. Their goal is to quickly move from tens of thousands of test compounds down to tens of compounds that might actually be effective and safe. In order to achieve this, high throughput screening (HTS) is cornerstone to primary testing, followed by secondary screens that are more precise and more closely replicate endogenous cellular conditions.
A plethora of screening techniques are at the disposal of research scientists, each with their advantages and disadvantages. Due to the very small scale of biological interactions within the body, scientists need to be creative in order to detect if test compounds have any effect on what they are studying. The majority of screening techniques rely upon some chemical label or tag in order to generate a detectable signal when binding does or does not occur to a specific probe. Current assays use fluorescence, chemiluminescence, or phosphorescence. While these have proven useful for HTS in primary testing, some disadvantages to all labeled assays are the expense of dyes or engineered cells, background noise, complicated assay design, and potential steric hindrance in binding. Also, these assays typically provide a low level of information that is very specific to one effect in one pathway. Labeled assays can provide an unrealistic picture of how test compounds would perform in vivo.
Label-free assays have received much attention for secondary screening, and technologies are being developed to increase throughput and sensitivity while reducing costs. Label-free assays have benefits over their labeled counterparts, such as increased sensitivity, increased biological relevance, and nontoxicity to cells. Biophysical methods include size exclusion chromatography, electrophoresis, NMR, mass spectrometry, and biosensors. Detecting target protein or receptor binding is strongly desired for drug discovery, which biosensors work very well for. A variety of signals can be measured by biosensor analytical devices, such as protein signals, acoustic and electrical signals, and light signals. Surface plasmon resonance (SPR) is a very useful technique that utilizes light signals to detect drug-target binding, reaction kinetics, mechanics, and thermodynamics while using a very small amount of test compound (on the order of micrograms of protein per reaction plate). This technology presents numerous advantages over alternative ones, and will be the focus of this paper. A condensed scientific explanation of how SPR works will be given first, followed by an overview of how SPR is being used in the drug discovery process.
Part 1 – Scientific Background
Surface plasmon resonance is a physical phenomenon of certain metals where upon excitation by incident light at the same frequency as surface electron oscillation (plasmons), a specific and unique resonance pattern is observed. Put more simply, a thin film of metal can absorb laser light and produce electron waves on its surface. This only occurs at a very specific angle of light and only at the thin metal-dielectric (water or air) interface. First looking at some underlying physical and chemical principles will help explain how SPR is possible.
Metals have some unique properties that are utilized with SPR. Foremost, metals are good conductors of electricity. This is because the atoms of metals are arranged together very closely and they are able to share electrons with each other. This is referred to as electron delocalization, or alternatively as a “sea of electrons.” (see Fig. 1) This property is is due to the configuration of the electron orbitals themselves. The electron orbitals represent probabilistic areas of where the electrons will be. The Aufbau principle gives a good approximation of what the electron configuration will be for most elements, but transition metals have significant exceptions to this principle. Electrons will seek the most stable, lowest energy configuration possible. Fully-filled orbitals or half-filled orbitals are more stable than partially filled orbitals, and can explain some of the strange behavior of electrons.
Figure 1 – Electron delocalization. A surface plasmon is characterized as a surface charge density wave at a metal surface. (Willets and Van Duyne, 2007)
One transition metal that is readily used in SPR is gold–an exceptionally stable metal which resists oxidation. The electron configuration of gold is [Xe] 4f14 5d10 6s1 and varies from the Aufbau principle by donating one of the 6s shell electrons to the 5d shell in order to fill it and achieve greater stability. The shape of s orbitals is spherical and extend outward in a radial fashion as the number of electrons increase. Electrons in gold’s outer 6s orbitals are incompletely filled, but because of orbital overlap, they are able to be shared by neighboring atoms and thus increase stability.
Electrons from the outer orbitals are still susceptible to manipulation, especially when the orbital is not completely filled. Electromagnetic radiation (EMR) carries bundles of energy called photons and is able to impart some of its energy on matter, as evidenced by microwave ovens. The wave-particle duality asserts that electrons have properties of both waves and particles. When the frequency of the incident EMR matches that of surface electrons on thin conducting metals, an amplification of this frequency will be propagated along the overlapping electron orbitals. This transfer of energy is referred to as evanescent-wave coupling, and it is necessary for SPR to occur.
Evanescent waves are formed when EMR undergoes total internal reflection at the boundary between two materials. Total internal reflection occurs when the angle of incidence is greater than the critical angle. When light is shined through a prism perpendicular to air (at 90 degrees, the normal), all of the light passes through and none is reflected. As the incident angle to the normal increases, more light is reflected back into the prism while the light passing through to air is refracted at a greater angle than the incident angle. This is dependent on the refractive indexes of the two materials, and in this case, air has a lower refractive index than the prism does. This continues until a critical angle is reached at which refracted light is parallel to the prism-air boundary. Angles greater than the critical angle achieve total internal reflection. This is the same concept which applies to fiber optics, and can best be described by Snell’s Law (n1sinƟ1=n2sinƟ2) and as seen in Figure 2a below. In addition to the total internal reflection requirement, the light has to be polarized parallel to the plane of incidence/diffraction (p polarized) or it will not be able to excite surface electrons (Fig. 2b).
Figure 2 – a) Total Internal Reflection. Based on Snell’s Law, light is refracted as is passes through different mediums, illustrated here as air and water. The refractive index for air is 1.0003 and for water is 1.333. b) Reflection of polarized light. Only light waves that oscillate in the same plane as the contact surface will be able to impart their energy on surface electrons. (source: Jossel7, Wikimedia Commons)
The theory behind evanescent waves is mathematically more complicated than this paper will investigate, but is important when determining the thickness of the thin film of metal (for more detail, see Willets and Van Duyne, 2007). Maxwell’s equations, which form the foundation of electrodynamics and optics, necessitate an additional wave is transmitted upon total internal reflection that carries no energy and does not travel but is non-vanishing. The only solution is waves that decay exponentially, or evanescent waves. This is observed experimentally through evanescent waves that penetrate up to one wavelength past the total internal reflection boundary, and are significantly more intense closer to the boundary (Marston, 2002). For light in the visible spectrum, this means a wavelength of around 1 um. Most SPR sensors have conducting metal thickness of 50 nm, fitting with these requirements.
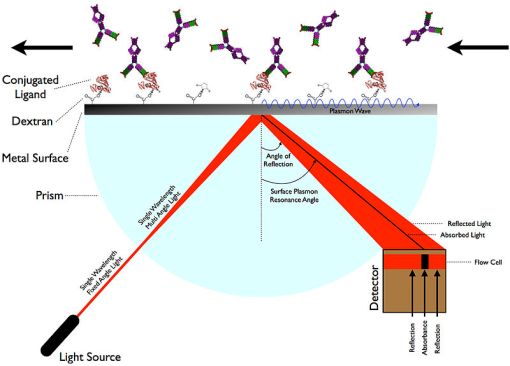
The resonance pattern is extremely sensitive to any changes to the environment just outside the thin film. Holding everything else constant, SPR will occur at a specific angle that changes when the refractive index of the buffer changes within one wavelength of the total internal reflection boundary. This angle is sensitive enough to differentiate molecular binding of up to 1.1 Angstroms in length (Ott et al., 1997). When a spectrum of light of different angles is incident upon a SPR chip (consisting of a metal surface and conjugated ligands), the specific angle at which SPR occurs will be detected as a dark band, indicating absorption. Practical applications of SPR use this method of spectroscopy to detect binding events. Analyte binding will alter the baseline dielectric environment. There are two main configurations for SPR but the Kretschmann setup is much more practical than the Otto setup. A typical configuration for SPR can be seen in figure 3 below.
Figure 3. Simplified diagram of a typical Kretschmann configuration for surface plasmon resonance. As binding events change on the alternate side of the metal surface, the SPR angle will change very precisely which is subsequently detected as a dark absorbance band. (source: SariSabban, Wikimedia Commons)
Part 2 – Practical Applications of SPR
New discoveries can take significant amounts of time in order to be fully realized by the greater scientific community. Such is the case with SPR. The first SPR immunoassay was proposed in 1983 when it was discovered (Liedberg et al., 1983). There are now numerous different applications of the technology and much progress has been made to reduce the cost of SPR analysis and increase the throughput. The benefits SPR has over alternative technologies will be discussed, followed by some examples of how SPR has been applied to drug discovery and molecular diagnostics.
Researchers should be quick to realize the numerous advantages SPR has over alternative technologies in the drug discovery process. Eliminating the need for molecular labels creates a microenvironment that much closer resembles the natural state in organisms. SPR can also determine molecular binding events in real-time. This technology can determine the binding and dissociation rates for particular compounds, as well as be used for competitive binding studies. Other applications include gene assays, protein conformational changes, environmental sensing, and electropolymerization.
A prominent example of utilizing SPR in a clinical setting comes from Alzheimer’s disease (AD). Small proteins called amyloid-beta derived diffusible ligands (ADDLs) bind to neurons, and when accumulated, these clumps of protein can disrupt normal electrical flow in neurons and damage them. While the disease remains complex, ADDLs still serve as relevant biomarkers for predicting disease onset. Using a sandwich type assay, researchers were able to use levels of ADDL relevant to in vivo conditions and obtain a more accurate characterization of oligomerization and fibrillogenesis. Other assays require significantly higher ADDL concentrations. This can be used to screen for drugs that block the oligomerization process, and thus provide a treatment for AD (Haes et al., 2005). Speaking more broadly, this demonstrates the ability of SPR to be used for diagnosis of any disease with an associated biomarker and ligand pair.
Cytochrome P450 enzymes are widely used in drug toxicity studies because they represent the major routes of metabolism for drugs, and interference to them can lead to drug concentrations below or above the safe, effective dose levels. Many techniques require a molecular reporter to determine binding to cytochrome P450s, but SPR can be used label-free to give a more accurate representation of molecular interaction. When specific cytochrome P450 enzymes are bound to the alternate gold surface, SPR can differentiate between enzyme substrates and inhibitors based on the shift in absorbance wavelength and determine the reaction kinetics (Zhao et al., 2008). This is useful for any research lab or company that is aiming to generate a safety profile for potential drug candidates.
Various SPR instruments are currently available on the market. Most–if not all–of these systems incorporate microfluidics. Because of the size limitations of such systems, all buffers must be degassed, passed through a 0.2 micron filter, and combined with 0.005% (m/v) surfactant P20 to reduce fouling of the inner surfaces. Cleaning steps are required to clear the system of remaining proteins and prevent bacterial growth. In order to prevent misinterpretation of SPR spectroscopy results, samples should first be dialysed or de-salted in the running buffer before testing to reduce changes to the dielectric constant (Manfield, 2004). Thus there exists great market potential for companies to make running SPR easier, faster, and more cost effective.
Companies continue to innovate with SPR technology to increase its value for drug discovery and molecular diagnostics. Long hailed as too expensive and too little throughput, companies like GenMark Dx are modifying SPR to confront these challenges. The basic technology behind their eSensor and XT-8 analytical system utilizes the same principles of SPR to detect specific DNA sequences in a patient sample. A polymerase chain reaction (PCR) amplifies the target sequence of choice, followed by a exonuclease reaction to create single stranded DNA, followed by a hybridization reaction with a small signal probe solution of complementary single stranded DNA. The XT-8 system utilizes microfluidics to simultaneously pump up to 24 different samples through channels containing a longer, complementary piece of single stranded DNA bound to a gold coating, whereby the instrument passes electrical current through the sample plates to record voltage changes. This is a molecular diagnostics testing system aiming to diagnose disease and optimize treatment. This technology is currently being applied in clinical settings towards a cystic fibrosis genotyping test, a warfarin sensitivity test, a thrombophilia risk test, and a respiratory viral panel, while HCV genotyping is still only at the research level.
Conclusion
Innovation does not happen in isolation or overnight. Often times it is the accumulation of pieces of knowledge from different fields that join together to create something truly unique and useful. New discoveries can have unexpected twists as they become assimilated into practice. What was originally described as a method to detect analytes in gas samples is now transforming the way disease is diagnosed and how drug candidates are screened and selected for optimization. The process has taken nearly 30 years to get to its current state, but it is clear that surface plasmon resonance is still a hot technology that continues to become ever more useful for researchers.
Works Cited
Haes, A.J., L. Chang, W.L. Klein and R.P. Van Duyne. (2005) Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J Am Chem Soc, Vol 127: 2264-2271.
Hartigan, Jennifer, Cindy Liu and William Downey. (2010) Moving forward with label-free technology. Drug Discovery World. Retrieved from http://www.ddw- online.com/screening/p142793-moving-forward-with-label-free-technology-winter-10.html
Liedberg, Bo, Claes Nylander and Ingemar Lundstrom. (1983) Surface plasmon resonance for gas detection and biosensing. Sensors and Actuators, 4: 299-304.
Manfield, Iain. (2004) Practice of surface plasmon resonance. University of Leeds. Retrieved from http://www.astbury.leeds.ac.uk/facil/SPR/spr_intro2004.htm
Marston, Philip L. (2002) Scattering of acoustic evanescent waves by circular cylinders: Partial wave series solution. Acous Soc Am J, 111-5, pp. 2378-2378.
Ott AW, Klaus JW, Johnson JM, George SM. (1997) Al2O3 thin film growth on Si(100) using binary reaction sequence chemistry. Thin Solid Films 292:135–44
Smith, Caitlin. (2009). Label-free Technology: A Myriad of Approaches. Retrieved from http://www.biocompare.com/Editorial-Articles/41666-Label-free-Technology-A-Myriad- Of-Approaches/
Thermo Scientific. (2012). Overview of Detection Probes. Retrieved from http://www.piercenet.com/browse.cfm?fldID=6AC86199-F81F-484C-B308-4EC4EB3F248F
Van der Merwe, P.A. (2001) Surface Plasmon Resonance in Protein-Ligand interactions: hydrodynamics and calorimetry, edited by S Harding and P Z Chowdhry. Practical Approach series, Oxford University Press pp.137-170
Willets, Katherine and Richard Van Duyne. (2007) Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 58:267–97
Zhao, Jing et al. (2008). Resonance Localized Surface Plasmon Spectroscopy: Sensing Substrate and Inhibitor Binding to Cytochrome P450. J. Phys. Chem. 112: 13084-13088


Assignment prompt: Research a technology and write a paper describing the assigned technology. The first part of the paper should explain the biophysical nature of the technology in a manner that can be understood by all students. The second part should explain how the technology is applied in drug discovery.
Grade: 82% “Did not cover SPR examples in enough detail, as the assignment included providing exemplary data.”